Titration Simulator to Learn Acid Base Equilibrium (Free)
This app uses the Henderson- Hasselbach equation and concepts in Acid-Base equilibria to do a simulation of a titration to determine concentration. You will be titrating of a strong acid (HCl) with a strong base (0.1M NaOH), or a weak acid (HCOOH) with strong base (1.0 M NaOH). The concentration of the acid will be unknown to you to simulate a lab experiment, but the volume will be fixed at 50mL. The pH indicator is phenolphthalein. This is a good way to do a dry run through a real titration experiment, before trying it at school!
Features:
- Simulate titration of strong acid (HCl) with strong base (NaOH), or weak acid (HCOOH) with strong base (NaOH).
- dynamic phenolpthalein visual indicator, along with pH and volume status
- dynamic droplet indicator
- adjust volume droplet between (0.2mL to 5.0mL), or simply tap faster
- Graph of pH vs volume of NaOH added shown at the end.
- Try to calculate the unknown concentration yourself, or click to Show Answer.
- Use this app to practice your titration skills, for when you need it in the lab.
- Suitable for first-year undergraduate or senior high school level students.
- Uses the Henderson-Hasselbach equation for the weak acid/ strong base combination.
- Calculates initial pH condition (no NaOH added), does not assume [A-] is zero.
- Switches to pH via pOH calculation when beyond equivalent point.
Instructions:
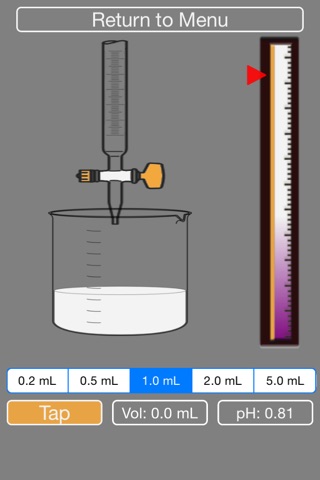
1. On the home screen, choose HCl or HCOOH to start the strong acid or strong base respectively.
2. You will be given NaOH in the burette at the appropriate concentration. Please select what volume of droplet you would like to add to the acid in the beaker (0.2, 0.5, 1.0, 2.0 or 5.0 mL)
3. Press Tap to add NaOH to the beaker.
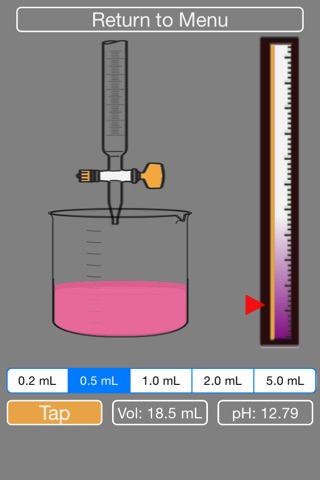
4. The volume of NaOH added and pH of current solution are indicated on screen. The pH indicator bar on the right shows the raising of the pH.
5. Press Tap faster to add more NaOH (this simulates leaving the stopcock open for longer.)
6. Tip: If using HCl, note that the acid will be neutralized very quickly. (strong acids dont make good buffers). Please use smaller concentrations, or else the pH will rise very quickly and you will miss the equivalence point.
7. Tip: If using HCOOH, you can use a higher droplet volumes first, then lower it.
8. For either acid, watch the fluctuation on the pH bar to regulate your droplet volume.
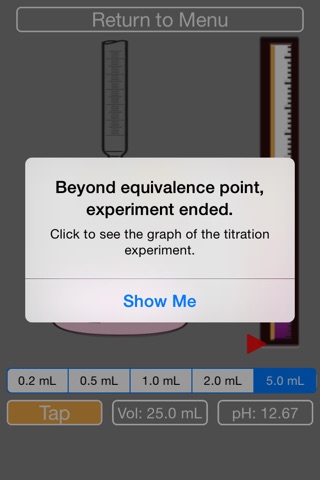
9. The acid will turn pink overtime, you need to keep adding NaOH until it turns fully pink (then the simulation will stop).
10. After the simulation stops, you are given the chance to see the titration curve. Y-axis is pH, and x-axis is volume of added NaOH.
11. Follow along on paper and try to locate the equivalence point by checking the center of the vertical line, and calculate the concentration of the unknown acid.
12. Or, the answer will be provided if you press ‘Show Answer’.